Data Access Committee
The Data Access Committee (DAC) is composed of members who have the expertise and skills in clinical research, data protection and management and research governance. The DAC Members will follow the DAC’s
Terms of Reference
and processes endorsed by the Precision Medicine Programme Board which adopt the Five Safes framework, and the broader principles of the Data Saves Lives policy paper. These principles enable data owners to provide safe and secure access to patient data to authorised and qualified individuals for purposes with a clear legal and ethical basis that is beneficial to the public and our local community.
Transparency
Barts Health are committed to being transparent to the patient and public regarding how their data is being stored, processed, managed, used and shared. To meet the legal and ethical requirements, in addition to capturing the views of the local community, Barts Health as the data controllers have implemented a DAC. To continue building public trust there are two public contributors on the DAC to ensure approved projects are aligned as to how patients and public would expect their data to be used. Data access requests approved by the DAC are publicly recorded, giving patients and public access to a record of how, who and why the community’s data is being used. In addition to basic information about the request, links to any publication and conclusions will be added as they become available, further demonstrating the benefit and impact of the research and use of data. View our approved projects.
Request access to data
Information on our standard research data sets can be found on HDR-UK and bespoke data sets can be provided upon request. A data access request can be made online.
The DAC aims to complete internal data approvals and establish projects within 2 months provided all external and required approvals from the Research Ethics Committee, Health Research Authority, Confidential Advisory Group and Joint Research Management Office (JRMO) sponsorship are in place. Please read the JRMO Standard Operating Procedures for further guidance. Barts Health is part of the EHDEN network and provides data in the OMOP data standard where feasible. Alternative data standard models and products can be supported depending on resource-constraints. Barts Health is committed to a user-driven approach to data standards to best meet the demands of our user community.
How to access the data:
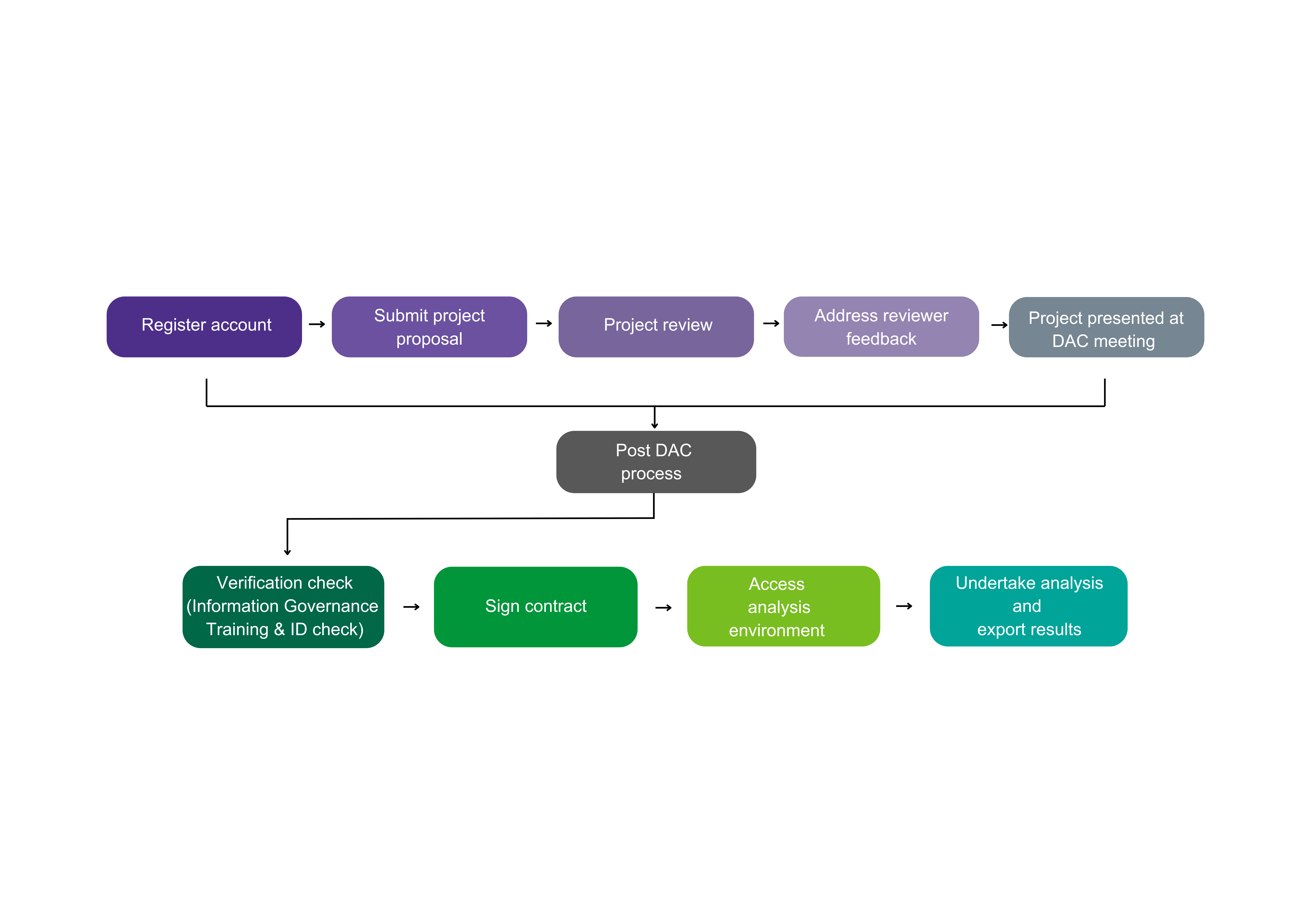
Watch our step-by-step video on how to request access here:
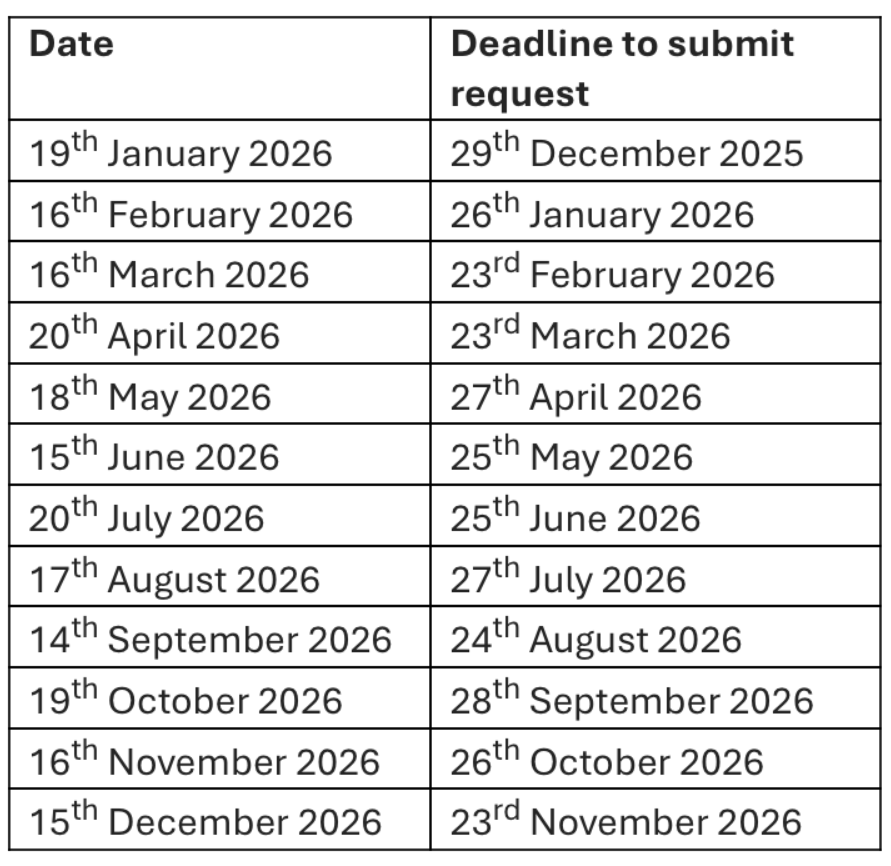
Upcoming meeting dates
DAC meetings take place on the third Monday of the month. Projects need to be submitted at least three weeks before the meeting to ensure there is sufficient time to complete the review process.
Acknowledgement
Publications arising from your use of the Barts Health Data Platform (BHDP) can be referenced through the paper - https://zenodo.org/records/13886268. The health data provided within the BHDP can be referenced through its meta-data - https://zenodo.org/records/10256449. Please also acknowledge Barts Health NHS Trust as the data and service providers and the funding by Barts Charity (G-001725& G-002196).


